DISCLAIMER: THIS IS NOT INVESTMENT ADVICE. I AM NOT A FINANCIAL ADVISOR. THIS IS FOR EDUCATIONAL PURPOSES ONLY. DO NOT USE THIS INFORMATION FOR INVESTMENTS --MAX HITEK
Be sure to visit Piotr Pietrzkiewicz's Patreon blog, "Bio_oko" , for many interesting articles related to drug development!
Notes On APOE4 and Potential EMA Blarcamesine Approval
2026 01/10 ---Max Hitek
Definitions and Abbreviations
Blarcamesine - - Anavex's drug, also known as A2-73.
AD - - Alzheimer's Disease.
ADAS-Cog - - The Alzheimer's Disease Assessment Scale-Cognitive Subscale. Used to assess the severity of the cognitive impairment. Considered to be the gold standard.
APOE4 - - Apolipoprotein E (APOE) E4. Human alleles are E4, E3, E2. E4 variant is the largest known genetic risk factor for late-onset AD.
MAB - - Monoclonal Antibody.
EMA - - European Medicines Agency
Discussion
As of this date, January 11, 2026, Anavex has not received EMA marketing approval for it's AD drug Blarcamesine.
Anavex is in the process of publishing some intriguing information from it's Blarcamseine trials1. This tanatalizing new data is not only surprising, but gives additional supporting reasons for approval.
Everyone carries two inherited alleles of the Alipoprotein E (APOE) gene. The two alleles will be some combination of APOE4, APOE3 and APOE2. The combinations that contain APOE4, correlate to an increased likelyhood of developing late onset AD. An individual can have either two copies of E4, or one copy, or none, with these approximate percentages:
E4/E4 E4/x x/x
15% 44% 41%
Anavex is publishing the following table in the paper:
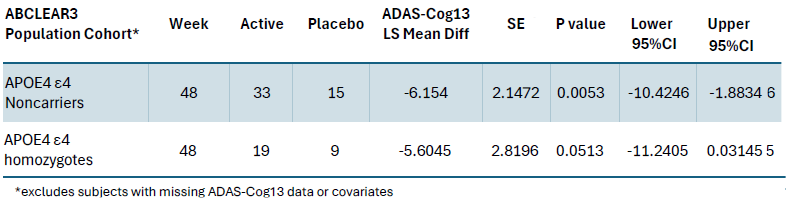
The interesting, and important column, is the ADAS-Cog13 column, which is the measurement of the cognition. It indicates that the group most likely to develop AD (E4/E4) homozygotes, have about the same cognition response to the drug as the group least likely to develop AD, the noncarriers. The implication is that patients are responding similarly well, whether they have the worst case of the APOE4 allele (E4/E4) or the best case, no E4. This is very unexpected and surprising.
Anavex stated, "This fact might be advantageous in geographic regions, where patients, who are APOE4 homozygous carriers, and hence are most in need for a treatment, are precluded from receiving recently approved injectable monoclonal antibodies."
This is a very interesting statement. We will break it down. Before we do that, we need to back up to the recently EMA approved AD drug Leqembi. Leqembi is a monoclonal antibody drug from the company Essai. Regarding Leqembi, the EMA published:
“... EMA’s human medicines committee (CHMP) has recommended granting a marketing authorisation to Leqembi (lecanemab) for treating mild cognitive impairment (memory and thinking problems) or mild dementia due to Alzheimer’s disease (early Alzheimer’s disease) in patients who have only one or no copy of ApoE4, a certain form of the gene for the protein apolipoprotein E."
When patients are dosed with Leqembi, the patients having having only one copy of APOE4, or none, are found to have less chance of having the serious side affects of brain bleed or brain swelling. The EMA does not want these side affects, so they do not want to Leqembi given to the E4/E4 group. Anavex, then, is saying that Blarcamesine does not have any serious side affects, particularly these nasty ones, so it would be especially applicable to the E4/E4 group. Keep in mind that Blarcamesine already appears to be much more efficacious than Leqembi.
Caveats
Here are the caveats. Anavex's table above, only cites data from their most responsive subgroup (ABCLEAR3). Is this efficacy seen for the whole intent to treat group, or another subgroup? It is not known. I believe these details will appear in another paper.
Conclusions
It appears that there are two additional reasons that Anavex could use to support the approval of Blarcamesine.
1.Blarcamesine cognition response is independent of APOE4 status.
2.Blarcamesine can be used where MABs are restricted because of patient E4/E4 MAB side affects.
References:
1Oral Blarcamesine Phase IIb/III Trial Confirms Identified Precision Medicine Patient Population – Significant Broad Clinical and Quality of Life Improvements for Early Alzheimer’s Disease Patient. September 29, 2025. by Macfarlane. www.medrxiv.org

